PT Interval Exceptions
The PT Interval Exceptions tab helps identify PT results where there is less than 7 days between the close of one study to the opening of the next. From 5.2.1.2 of the TNI standard:
- The opening date of PT study samples for a particular field of accreditation must be at least seven (7) calendar days after the closing date of a PT study for the same field of accreditation.
- A laboratory that analyzes and reports PT study results with an opening date of subsequent PT studies for the same field of accreditation that are closer than seven (7) days from the closing date of the previous PT study are invalid for the purposes of compliance with this Standard and are not counted toward the laboratory’s PT history of the most recent three (3) attempts.
AB Manager’s PT Summary table indicates, for a specific matrix, analyte, and technology (see Note 1), the most recent 3 PT Studies (with corresponding dates, evaluation, and methods used). Study 1 is the oldest and Study 3 is the most recent.
The PT Interval Exceptions table displays any PT data where the time between studies is less than 7 days, specifically:
- Close 1 to Open 2 is <7 days, or
- Close 2 to Open 3 is <7 days.
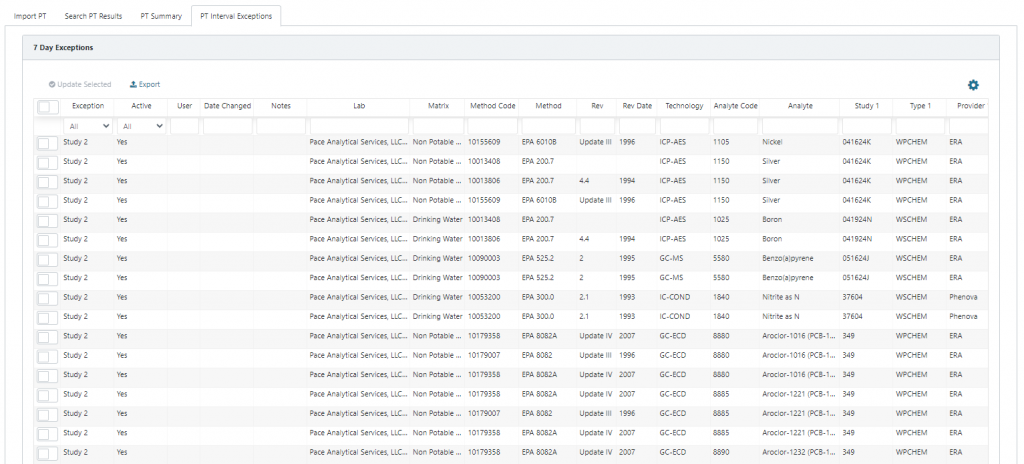
If PT interval exceptions are reviewed and determined to be invalid, the user may select those results, select update selected, and then change Active to No. Marking PT results as inactive will remove them from evaluation in the PT Summary. PT data marked as invalid can be reverted to valid in the Search PT Results table.
Note: To revert results back to Active, use the Search PT Results table to find and update the active status.
Note: AB Manager’s ‘PT Summary’ and ‘PT Interval Exceptions’ tabs group PT results by technology as per TNI 4.3.4. As a result, when multiple methods with the same technology are reported, there may be cases where a single method may have an acceptable PT internal but may fail as a technology group. These exceptions must be evaluated manually.
Example
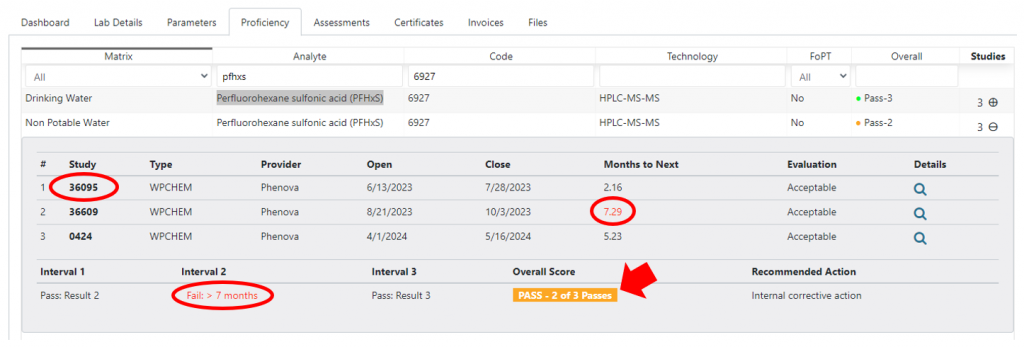
In this example, the PFAS analyte Perfluorohexane sulfonic acid (PFHxS) is identified as a ‘Study 2’ PT interval exception. Using the laboratory Proficiency tab I can display the most recent 3 studies for any given matrix/analyte/technology combination. In this example, we can see that:
- The study 2 (1023) open date is one day before the close date for study 1 (36609), therefore study 2 is considered invalid.
- The PT summary result is ‘3 of 3 passing.’
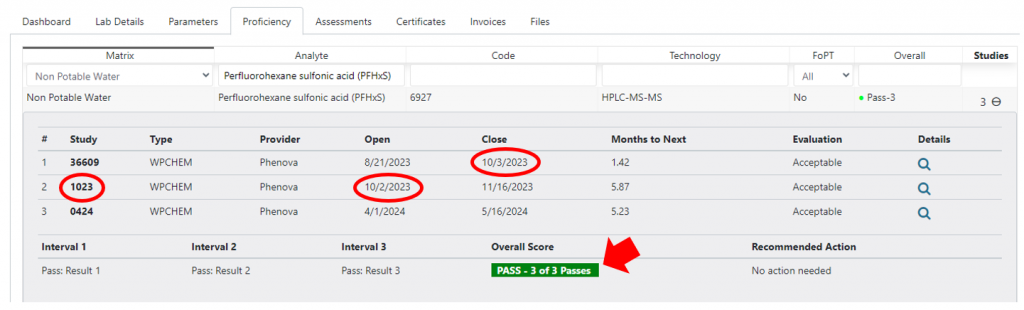
Using the PT Interval Exceptions table, the user can select invalid data, change active to ‘no’, and add notes. Inactivated PT data will not be used in the PT Summary tables. In this case, study 1023 is removed and study 36095 becomes the third study:
- There are no longer any interval exceptions.
- There is now an exception indicating that the close date of study 2 to the open date of study 3 is greater than 7 months.
- The PT summary result is ‘2 of 3 passing.’