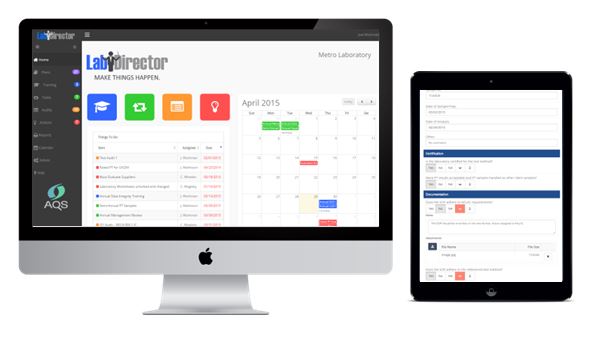
QA Manager Quality Management System
QA Manager Quality Management System is an innovative and powerful application for tracking and maintaining your organization’s ongoing quality, environmental, health, safety, laboratory and other compliance requirements. LabDirector makes it easy to stay on top of one time and recurring requirements. Never miss another deadline – you’ll be better prepared than ever for your next audit or inspection. Lab Director helps bring teams together to make plans, delegate responsibilities, review progress and resolve problems. Five powerful modules work seamlessly together to make sure nothing slips through the cracks.
Frequently Asked Questions
 Quality management is the pursuit of excellence – striving to meet the needs and expectations of customers. In today’s competitive marketplace, quality is the difference between success and failure. Consistent quality takes effort, planning and follow through.
Quality management is the pursuit of excellence – striving to meet the needs and expectations of customers. In today’s competitive marketplace, quality is the difference between success and failure. Consistent quality takes effort, planning and follow through.
Compliance management is the process by which managers plan, organize and control activities that ensure compliance with regulations, laws and standards. For example, strict regulations exist for environmental, health & safety, food handling and other areas. Failing to comply with regulatory statutes and standards can have costly consequences.
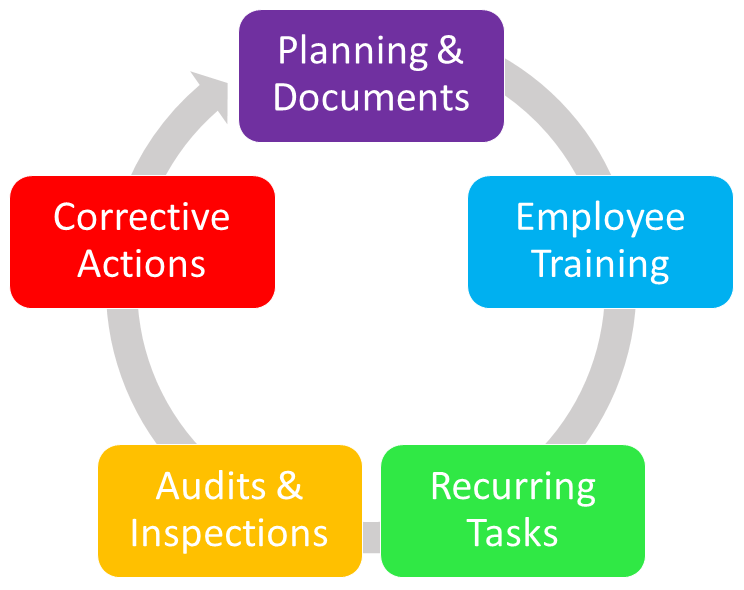
The key elements of a quality and compliance management system include:
- Planning and documentation
- Employee training and improvement
- Recurring tasks
- Internal audits and inspections
- Corrective actions and continual improvement
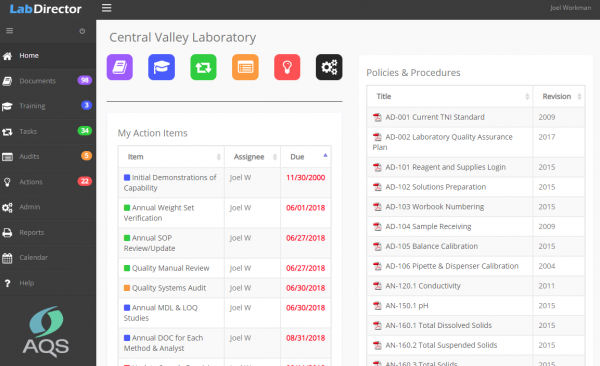
Lab Director is an effective and easy-to-use quality and compliance management application. Five integrated modules help ensure that every internal and external requirements is considered, delegated, completed and documented.
Lab Director is an excellent tool for defining, delegating, and tracking specific assignments and responsibilities. For each requirement, admins or managers designate the title, description, start and end dates, person(s) responsible, recurrence pattern and other specifics. In Lab Director, this creates discrete “instances” of that assignment. On the start date for each instance, that assignment will appear on the individual and team Action Items list. Action items are prioritized by due date with warnings for overdue assignments.
LabDirector QMS can replace at least five different programs or calendars that you’re currently using to:
- Coordinate action items and assignments,
- Control and distribute documents,
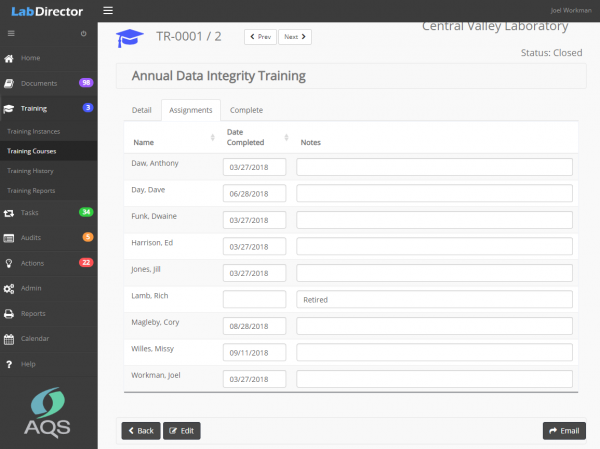
- Track employee training,
- Delegate and follow-up on recurring tasks,
- Conduct on-site audits and inspections, and
- initiate and complete corrective actions.
AQS is a technical consulting company and we use Lab Director every day to help our clients stay in compliance with internal and external requirements. We are always adding new features that matter to our clients. Lab Director’s key features include:
- Web-based design, accessible anytime, anywhere
- Prioritized task lists for individuals or teams
- Daily email notifications
- Recurring tasks with start and due dates
- Optional review step for critical tasks
- Upload photos, documents or other attachments
- Assign individuals or groups to specific tasks
- Complete and save inspections on a laptop or tablet
- Easily download or build customized inspection forms and checklists
- Document and delegate corrective and preventative actions
Lab Director is a web-based application accessible from any web-enabled desktop, laptop, tablet or cell phone. Every feature is completely portable. For example, an inspection form can be built on the office desktop and then completed in the field on a laptop or tablet.
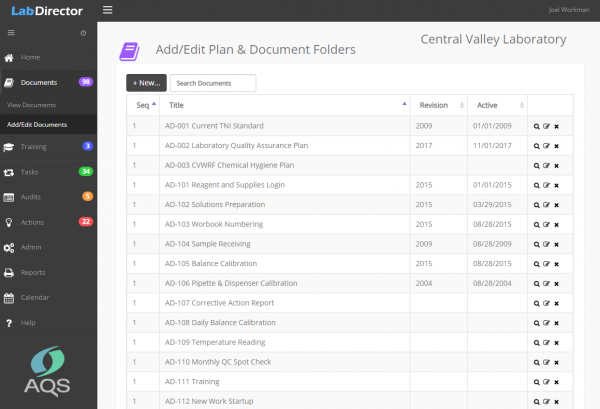 Planning and documentation are the centerpiece of an effective quality and compliance management system. Plans and procedures should be readily available to employees and only the current version of a document should be used. In addition, all previous document versions should be maintained and accessible if needed.
Planning and documentation are the centerpiece of an effective quality and compliance management system. Plans and procedures should be readily available to employees and only the current version of a document should be used. In addition, all previous document versions should be maintained and accessible if needed.
Lab Director is a very effective online document management system. Managers create a folder for each document and revisions are uploaded to that specific folder. Each revision includes the date added, date active and revision number. Only the current version of each document is displayed and available on n the Lab Director home page. By organization policy, all printed copies of the document are considered to be uncontrolled.
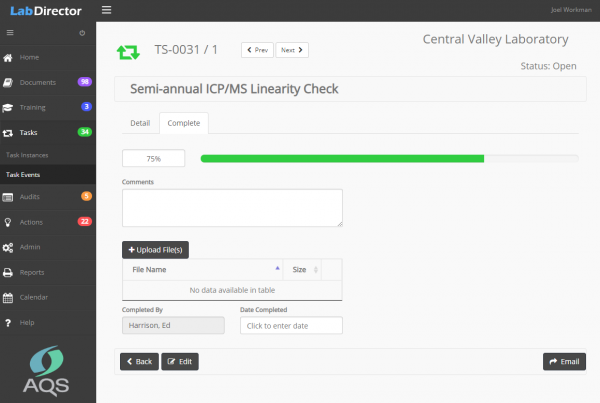 Every business or project has important tasks that must be completed on a regular basis – weekly, monthly, quarterly, annually. Lab Director is much more than a calendar or task management program. Prioritized tasks show up only when you need them and they can be assigned to anyone on the team. Completed tasks, notes and attachments are saved for inspection and review.
Every business or project has important tasks that must be completed on a regular basis – weekly, monthly, quarterly, annually. Lab Director is much more than a calendar or task management program. Prioritized tasks show up only when you need them and they can be assigned to anyone on the team. Completed tasks, notes and attachments are saved for inspection and review.
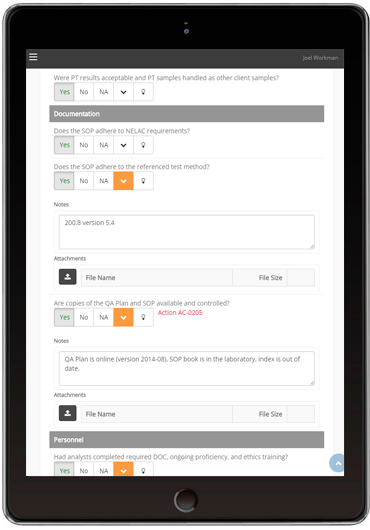 Regular audits and inspections ensure that internal and external quality and compliance requirements are being followed. Checklists ensure that audits are thorough and consistent.
Regular audits and inspections ensure that internal and external quality and compliance requirements are being followed. Checklists ensure that audits are thorough and consistent.
The Lab Director Audit module makes it easy to build checklists, conduct inspections and generate reports. Required inspections can be assigned, scheduled, recorded and shared within the application. Existing paper checklists can be easily converted to our online checklist. Commonly-used checklists can also be downloaded from our online library. Inspections forms can be accessed from any web-enabled device – including a tablet or mobile phone. Inspection details, including photos or videos, can be attached to any checklist question. Corrective actions can be generated directly from the application.
How Easy is it to Create My Own Checklist? Super easy – just edit, upload and go. A checklist template is created or edited using Microsoft Excel of similar CSV editor. The checklist design and details are completely customizable to include:
- Section headers
- Categories
- Notes
- Questions with Yes, No and NA
- Photos (phone or tablet only)
- Attachments
- Text
- Date
- Signatures
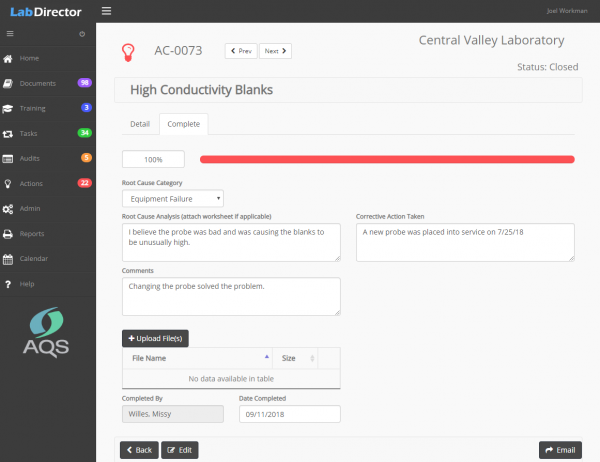 Corrective and preventative actions are an excellent way to fix problems and improve the process. Formal corrective actions allow your organization to follow up on internal audits, external audits, customer complaints, or staff suggestions.
Corrective and preventative actions are an excellent way to fix problems and improve the process. Formal corrective actions allow your organization to follow up on internal audits, external audits, customer complaints, or staff suggestions.
To complete the action, a root cause must be identified and a proposed corrective action. Supporting documentation, photographs and notes are attached. The corrective action remains on the Action Items list until it is completed and reviewed.
AQS offers flexible pricing based on your organization’s needs. Please contact us at 801-476-1365 for more information.